

In the Lewis dot diagram for CO2, two Oxygen atoms will now form double bonds with a Carbon atom, completing the Lewis structure. A double bond is therefore formed between each Oxygen atom and the center atom.ĭouble bonds between Oxygen and Carbon atoms may now be shown by drawing two parallel lines connecting them. Since the octets of two oxygen atoms each need two electrons, the two electrons from the carbon atom are shared to create double bonds. In this case, Carbon will transfer its electrons to both of these Oxygen atoms since Oxygen is more electronegative than Carbon. This may be accomplished in one of two ways: by giving or receiving an electron. In addition to the location, two Oxygen atoms on each side of the atom, and six dots surrounding each atom symbolize the valence electrons.Ī molecule may become stable and inactive by attaining an electrical state comparable to that of inert gases by completing its octet. So, for the time being, put Carbon in the middle and circle it with four dots. Let’s look at the valence electrons of all the atoms in the molecule to see how the bonds are formed and how they are organized. Carbon atoms share electrons with the two Oxygen atoms on the terminals and link to each other. The Carbon atom has the most prominent position in CO2 because it is the molecule’s least electronegative atom. Here is the Lewis dot diagram for CO2 to show you how it is drawn and how it is used.
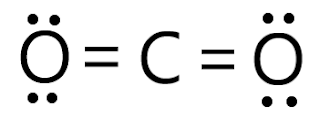
Understanding the arrangement of atoms and electrons involved in the bond formation is made easier by this structure. The molecule’s bonds are shown by lines drawn on the surface of the molecule. As a result of the Lewis dot structure, the valence electrons may be seen by placing small dots around the atoms. The valence shell electron configuration of a molecule is shown pictorially by the Lewis dot structure. Before learning about the Lewis dot diagram for CO2, it is necessary to have a firm grasp of what constitutes a Lewis structure. To better understand the arrangement of electrons in molecules and their form, this structure is useful. Oxygen is a nonpolar diatomic molecule with a 180-degree bond angle.īoth oxygen atoms in the molecule have the same electronegativity value, and both atoms share identical ratios of bound shared electrons, making the O2 molecule nonpolar in nature.Any molecule’s molecular geometry cannot be understood without first having a firm grasp of the Lewis structure. In the O2 Lewis structure, there is a double bond between two oxygen atoms. They can be identified by using a Lewis structure. Lone pair of electrons are found in the outermost electron shell of atoms. The lone pair of electrons are unshared valence electrons. Oxygen also possesses a lone pair of electrons. Similarly, to complete its octet, oxygen shares three valence electrons with carbon. By sharing three valence electrons, carbon forms three tipple bonds with oxygen. The overall carbon to oxygen atom ratio in a CO dot structure is 1:1.


 0 kommentar(er)
0 kommentar(er)
